ICER unveils latest report on 'unsupported' drug price increases
The drug pricing watchdog known as the Institute for for Clinical and Economic Review on Monday unveiled its annual report on what it says are drug price increases unsupported by new clinical data.
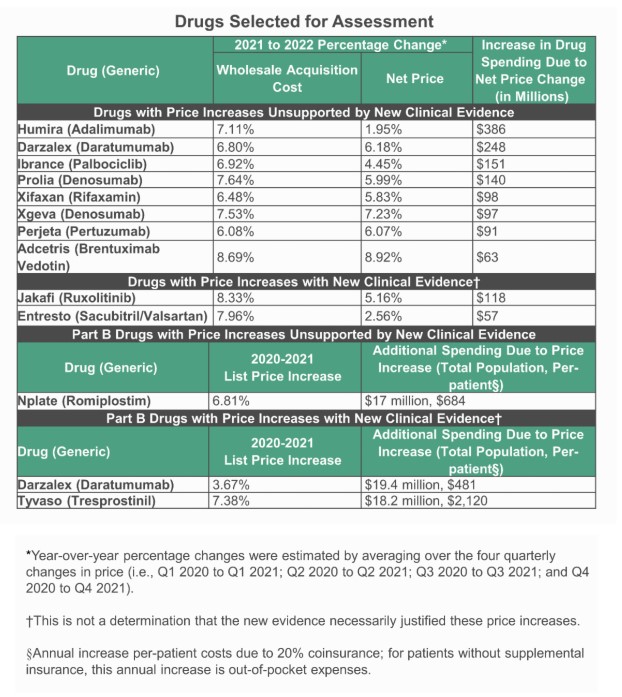
But some in the industry are pushing back on the report, which pulls data from 2021 and 2022. For AbbVie’s megablockbuster Humira (adalimumab), for instance, biosimilar competition has driven the price down significantly since these increases were made.
 David Rind ICER
David Rind ICER ICER said it selected its 10 drugs for the 2023 list from the top 250 drugs by 2022 US sales revenue and for which wholesale acquisition cost (WAC) price increases that exceeded the consumer price index, plus 2%. The drugs that made the top 10 list had net price increases (vetted by manufacturers) that contributed to the largest increases in US spending.
ICER said these increases among the top 8 accounted for $1.27 billion in additional costs over one year, which was comparable with years past.
“We continue to see list price increases above inflation for many of the most costly drugs,” David Rind, ICER’s chief medical officer, said in a statement. “When we look further at those drugs whose net price increases led to the largest increases in US expenditures, many had no substantial new evidence to support such price increases.”
But others in the industry have raised questions about this particular report and its relevance.
“This is a report that should be discontinued for a number of reasons,” John O’Brien, president and CEO of the National Pharmaceutical Council, told Endpoints News in an interview. “Old data that contains methodological shortcomings really does a disservice.”
Another important piece missing from the report: CMS rebates — required to be paid to the government for any drug for which the price rises faster than inflation — went into effect in January 2023, just after the data from the ICER report cuts off.