Fake air cleaners, jaundice scanner, Yet Another Lyme Vaccine, and more
18 Nov 2021
Posted by Andrew Kantor
Georgia State is ready to track Covid-22
When the next pandemic appears, why turn to your favorite news site to track it, when Georgia State computer-science and maths researchers* have created software to do it?
When someone gets sick, epidemiologists upload the gene sequence of the virus to a global database, where it can be analyzed. But current software is slow, while Georgia State’s SPHERE (Scalable PHylogEny with Recurrent mutations) “can process more than 200,000 novel virus genomes in less than two hours.” That lets scientists, and in theory politicians, make smart decisions based on how the virus — and its mutations — are spreading.
* You either thought “nerds” or you’re lying about thinking “nerds.”
Coffee continues to be good for you
Part of me is tired of “coffee is good for you” stories, but part gets a kick out of how often they appear*. So here: “Drinking tea and coffee could lower your risk of stroke and dementia, study suggests.”
Following more than 360,000 patients over more than a decade…
Researchers found those who drank two to three cups of coffee or three to five cups of tea or a combination of both for four to six cups a day had the lowest risk of dementia or stroke.
Why? They aren’t sure. Could be the caffeine, or maybe the polyphenols or the flavonoids, or “the combined protective role of the different antioxidant and other biological contents in these 2 beverages.”
Fun fact: Digging deeper into the paper itself, though (and assuming I’m reading it correctly), ground coffee is better than instant, instant is better than decaf.
CDC’s got the latest on Covid shots
In case you’re interested, the CDC has updated its what-you-need-to-know site about Covid vaccines with the latest info for giving the shots to kids.
Biosimilars: Copies of copies
Biocon/Viatris are launching not one but two versions of Semglee, their biosimilar for Lantus. (Semglee made news in July because it’s the first interchangeable biosimilar — pharmacists don’t need approval to swap it out for Lantus.)
Weirdness: There are two versions, one branded (Semglee) and one unbranded (“insulin glargine”). So yes, there’s the branded drug (Lantus), the branded biosimilar (Semglee), and the ‘generic’ biosimilar. This will be on the final.
Why two versions of an identical identical drug? Pricing!
By launching two versions of the same product, Viatris is effectively providing payers with the option to choose between a high price, high rebate product [Semglee], or another that carries a lower price and features a lower rebate [“insulin glargine”].
We wish you luck explaining this to patients and dealing with insurance companies.
Another Lyme disease vaccine in the works
This one uses mRNA technology, and it targets the ticks. No, you don’t have to vaccinate the bugs — the host (i.e., eventually you) gets the shot, and that turns you into a tick-killing machine.
A Yale infectious disease researcher, collaborating with folks in Ireland, gave his experimental vaccine to guinea pigs. Sure, the bites turned red, but “The ticks fed poorly, fell off early, and often failed to transmit the Lyme-causing bacterium.”
Human testing is coming.
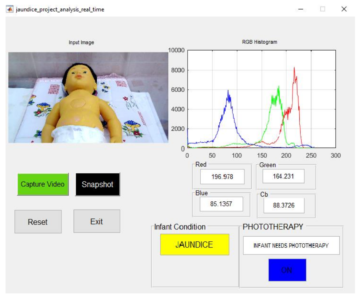
Scanning for jaundice
A new system can detect whether a newborn has jaundice, start treatment (blue/turquoise LEDs), and notify a caregiver — all within one second.
The device, developed by Aussie and Iraqi medical engineers, is non-invasive: It analyzes images of the baby in real time and is sensitive enough to detect a color change invisible to the human eye. And it works no matter the pigmentation of the child’s skin.
Stop with the extra BP meds
Giving hospitalized patients more or a higher dose of anti-hypertension meds is common — and dangerous. So argues a Duke University physician, who points out that just being in hospital can make someone’s BP rise.
For example, acute pain ramps up your sympathetic nervous system, causing your blood pressure to rise. In such a situation, receiving new blood pressure medicine increases the chance that a patient will need to be readmitted to the hospital in the 30 days after discharge, probably because their blood pressures crash once they return home.
Patients: If you do get a new BP med in the hospital, ask whether you need to keep taking it at home. Or keep track with your own sphygmomanometer (which also lets you brag to friends, “I have a sphygmomanometer … and I know how to use it”).
Atlas twitched
Well this is embarrassing. Schools across the country — from kindergarten through university — used Covid funds to update their air-cleaning systems. Better air circulation reduces the risk of transmission, so it was a smart idea.
Well, smart as long as the companies selling those systems didn’t lie about them.
Oops.
School districts and universities have spent as much as $100 million on this technology — often for electronic air cleaning systems that have misleading, company-funded studies that boast 99.99% efficacy,
Seems that, when products aren’t subject to annoying regulation, companies flat out lie about them. As one engineer quipped, “99.99% doesn’t mean anything if you’re testing in a shoebox.”
Warnings are being issued (to the schools), lawsuits are being filed (against the companies), but in many cases the schools’ only option is to cancel existing orders. Sorry, kids.