FDA learns to smile, plus smog psychosis, planting a cholera vax, and more
28 Aug 2021
Posted by Andrew Kantor
Missing numbers
How bad is Covid in Georgia? We can’t know for sure because, as Georgia Health News reports, “Two state government websites in Georgia recently stopped posting updates on Covid-19 cases in prisons and long-term care facilities.”
Other states, too, (notably Florida) have cut back on reporting cases in the middle of this fourth pandemic surge.
Pharmacists should prophylactize
A group of 12 pharmacy organizations, including APhA, NCPA, and NASPA, have asked the FDA to allow pharmacists to provide the REGEN-COV monoclonal antibody Covid-19 treatment.
The FDA gave emergency use authorization to REGEN-COV (casirivimab and imdevimab) as a post-exposure prophylaxis for Covid. If it’s given when symptoms first show, but before hospitalization or oxygen therapy, it seems to make a difference for high-risk patients.
Time, they point out, is a major factor.
The groups indicated that when patients access care from a pharmacist, directing the patient to another provider for initiation of prophylaxis would unnecessarily delay treatment, if post-exposure prophylaxis occurs at all.
The city mouse may be a little … off
…at least compared to the country mouse. A new British study found that the higher the level of air pollution in an area, the more people have to be treated for psychotic and mood disorders.
A stem-cell diabetes treatment
A theoretical way to cure diabetes would be to take stem cells and turn them into pancreatic beta cells, then put back into the patient.
Problem 1: There are a limited number of human stem-cell lines available. Problem 2: The process of turning stem cells into the cells you want is expensive … and doesn’t always work.
Enter Penn State biomed engineers. They’ve come up with an inexpensive and easier way to differentiate those stem cells into pancreatic cells (solving problem 2). But their method also works on stem cells that are made from a patient’s own mature cells (solving problem 1).
Take cells from patient. Turn cells into stem cells. Use new method to turn stem cells into pancreatic beta cells. Put into patient. Celebrate.
Well, that doesn’t work either
The latest hopeful Covid-19 treatment that has flopped in testing is a biggie: convalescent plasma. As explained in a paper in the New England Journal of Medicine…
The researchers hoped the treatment would reduce disease progression by 10%; instead the reduction was less than 2%. Though researchers conceded that the treatment might be effective in certain other cases, such as preventing symptoms after exposure.
Another big step toward a malaria cure
The standard malaria treatment around the world is piperaquine plus dihydroartemisinin. And it works … okay. But Purdue chemists have just completed a phase 2 clinical trial: It shows that adding imatinib (aka Gleevec) to the mix “enables clearance of all malaria parasites from 90% of patients within 48 hours and from 100% of patients within three days.”
Oh, and cholera, too
Not a cure, but a new vaccine: This one is grown in rice, which is then crushed into a powder, mixed with saline solution, and then drunk.
It has huge advantages over pills: It’s easy to make (no more bioreactors), has a three-year shelf life (not just 14 days), and is cheaper to produce — and it may last longer than current vaccines because it targets the cholera toxin, not the cholera bacteria.
Tests are harder to come by
CVS says it’s limiting sales of at-home Covid-19 tests (a max of six online, four in person) because demand has shot up. Supply, though, is low because test makers slowed production during the summer when they figured — like most of us — that the pandemic was winding down.
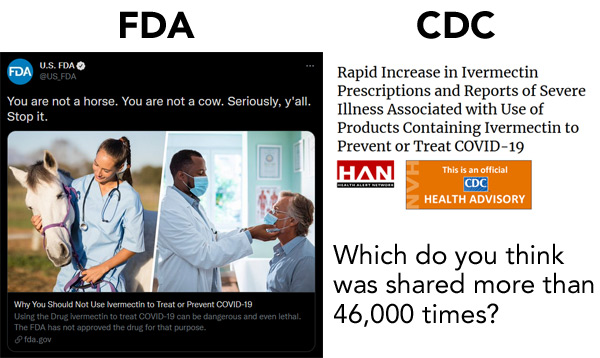
CDC issues ivermectin warning
The agency released an official health advisory — sorry, “HEALTH ADVISORY” — warning about the dangers of ivermectin, even when it’s by prescription: “Rapid Increase in Ivermectin Prescriptions and Reports of Severe Illness Associated with Use of Products Containing Ivermectin to Prevent or Treat COVID-19.”
You don’t say
The FDA has apparently discovered what we at GPhA Buzz have been saying for years: When you mix a little humor into your news, people pay attention.
“We hope that people will come for a little bit of snark, stay for the serious, and in the process learn something new about the FDA and an issue that could save their lives.”