When acne is your friend, why CVS is doing biosimilars, getting yeast to make drugs, and more
25 Aug 2023
Posted by Andrew Kantor
CVS launches biosimilar company
CVS Health has partnered with Novartis to produce a biosimilar of Humira, which it says it will price at 80% below Humira’s $6,922 per month price. It’s created a new company called Cordavis to do that .
Other companies also make Humira biosimilars, but some of them price them close to Humira. Why? Because PBMs like high drug prices — high list prices mean high kickbacks rebates.
But Cordavis will price its biosimilar (“Hyrimoz”) a lot lower. Why? So CVS Caremark (the PBM) can buy it cheaply and thus offer it to CVS pharmacy patients at a low price, but without dealing with manufacturer rebates.
In other words, a PBM is cutting out the middleman and making the drug itself.

The world of PBMs and drug pricing (artist’s conception)
At this point you’re permitted to hit yourself on the head and hope this makes sense. (It does, but only in the crazy world of PBMs.)
Why else is CVS doing this? Because Mark Cuban Cost Plus Drugs is also making a Humira biosimilar, and selling it directly to patients for $569 a month.
Region meetings teaser
Psst! GPhA’s region meetings are coming soon — in late September for most of you. The details are still being finalized, so that’s why this is just a teaser. More details to come!
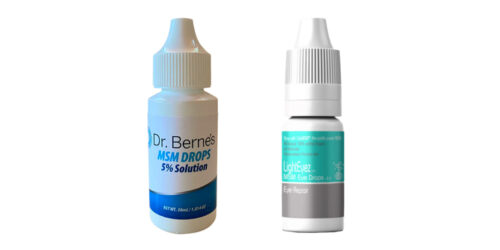
Eye drop warning
The FDA says people should immediately stop using and throw out any bottles of “Dr. Berne’s MSM Drops 5% Solution” or “LightEyez MSM Eye Drops – Eye Repair” — they could be contaminated with bacteria, fungus, or both.
Acne bacteria is trying to do good
It seems that the bacteria that causes acne and angst among teens (Cutibacterium acnes) might actually do more than ruin prom night for a few kids. UC San Diego researchers found that it does a lot to protect the skin by stimulating lipid production.
We found that C. acnes induced this increase in lipid production by producing a type of short-chain fatty acid called propionic acid. Propionic acid creates an acidic skin environment that provides a number of benefits, including limiting pathogen growth, reducing staph infections and contributing to anti-inflammatory effects in the gut.
In fact, they said, only this bacteria has these effects, meaning Clearasil sales are just a happy byproduct of the body protecting itself.
FDA smacks Exeltis upside the head (gently)
Perhaps drug maker Exeltis figured the FDA was too old to be on social media, so it tried to slip in an ad for its Slynd contraceptive without clearing it with the agency first. That’s a no-no.
And then a no-no-NO was leaving out some important info from that ad.
According to the FDA, the post lacked any information about the risks of Slynd, despite the label of the oral contraceptive describing warnings and precautions related to high potassium, blood clots, bone loss, cervical cancer, and more.
And a no-no-no-NO was making the info that was in the ad misleading — saying it would give women “periods on a schedule” when the company has no data to support that.
And thus the FDA sent Exeltis a strongly worded letter telling it to stop doing that.
New bacteria, new antibiotic
Did you know that 99% of bacteria can’t be cultured? Well, couldn’t be, until a small US startup figured out how.
So what? What: That means there are suddenly a lot more bacteria that can be “mined” for new antibiotics. It’s not just theory, either; for the first time, scientists were able to isolate and culture a bacteria called Eleftheria terrae in which they discovered a new antibiotic: Clovibactin.
“Since Clovibactin was isolated from bacteria that could not be grown before, pathogenic bacteria have not seen such an antibiotic before and had no time to develop resistance.”
Even better, Clovibactin’s mechanism is different than existing antibiotics, so, the scientists say, “bacteria will have a much harder time developing any resistance against it.”
The Long Read: FDA commish on shortages
Why are drug shortages hitting so hard and so fast? FDA commish Robert Califf thinks it’s a two-pronged problem.
The “fundamental problem,” to hear Califf tell it, is that “we essentially have two drug industries in the U.S.”
There’s the “innovator industry,” where Califf said he thinks “the prices are too high,” and then there’s the generics industry, where “a lot of the prices are too low.”
Fierce Pharma has the details.


